ISO 13485 is an internationally recognized Quality Management System (QMS) standard specifically designed for organizations involved in the design, development, production, installation, and servicing of medical devices. The standard focuses on meeting regulatory requirements and ensuring the consistent safety and effectiveness of medical devices throughout their lifecycle.
ISO 13485 is aligned with global medical device regulations and supports compliance with international regulatory frameworks, including requirements related to risk management, product traceability, and regulatory documentation.
Adopting ISO 13485 demonstrates an organization’s commitment to patient safety, product quality, and regulatory compliance. A compliant Medical Device QMS ensures that quality and regulatory requirements are systematically planned, implemented, monitored, and improved across all processes.
ISO 13485 emphasizes risk-based thinking, documented controls, and process consistency to ensure medical devices meet both customer and regulatory expectations.
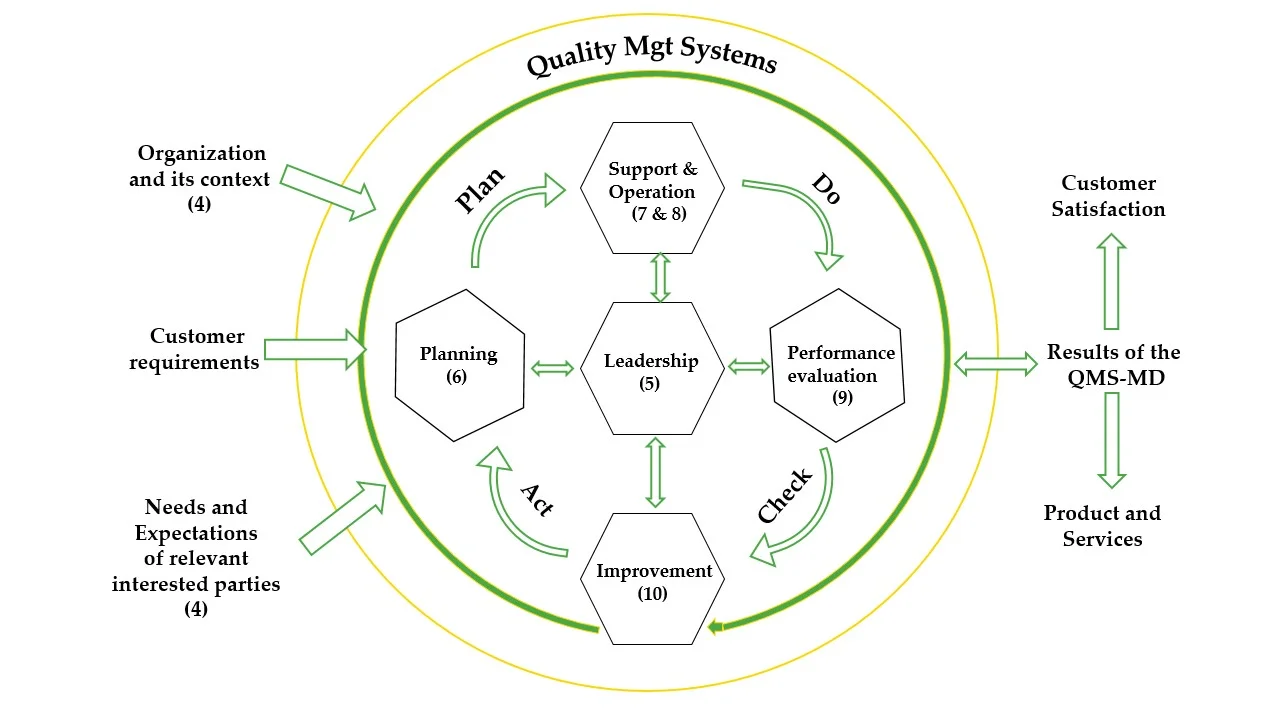
In the medical device industry, quality failures can lead to patient harm, regulatory penalties, recalls, and reputational damage. ISO 13485 provides a robust framework to manage these risks and maintain compliance with applicable medical device regulations.
ISO 13485 enables organizations to:
ISO 13485 certification is often a mandatory requirement for market access in regulated medical device markets.
Operating with a certified Medical Device QMS enables organizations to:
ISO 13485 certification provides assurance that quality and regulatory controls are effectively implemented and maintained.
ISO 13485 certification demonstrates that an organization:
By adopting ISO 13485, organizations build trust with regulators, customers, and patients while supporting sustainable growth and global market access.
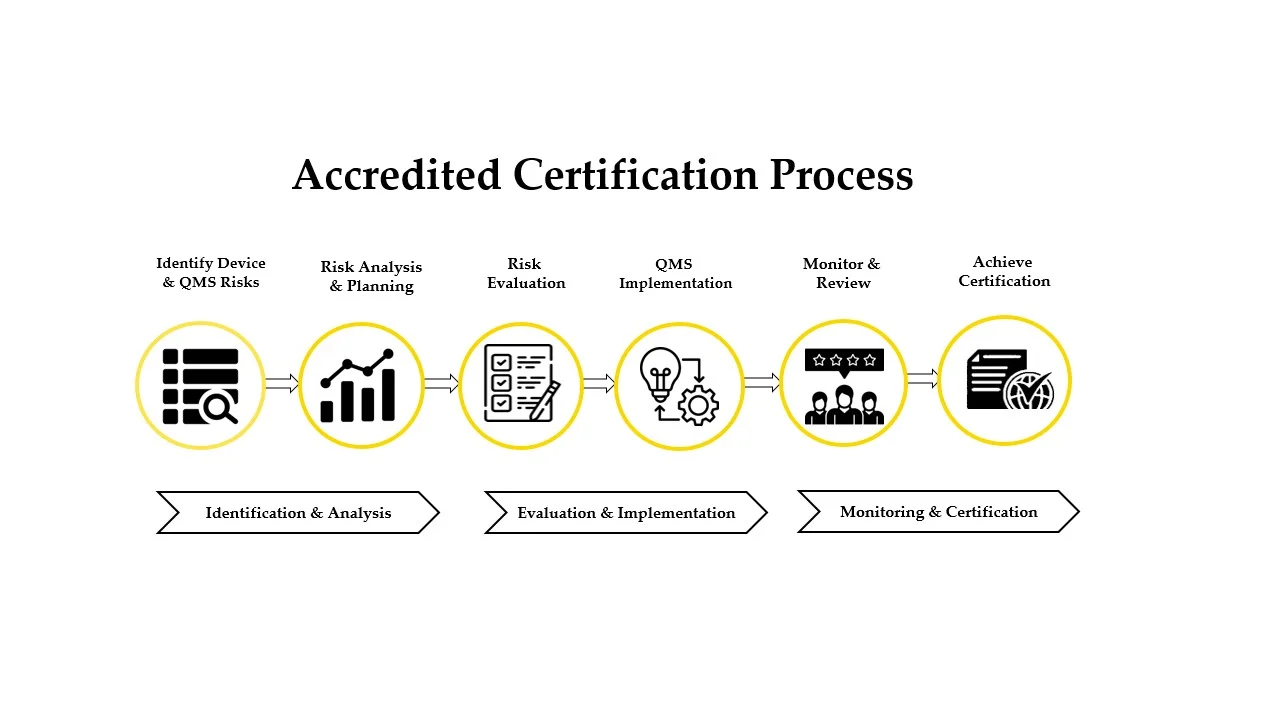
Strengthen credibility, meet international compliance standards, and build trust with customers and stakeholders through globally recognized ISO and compliance certifications.
TESTIMONIALS
Excellent training! The blend of theoretical knowledge and hands-on application elevated my auditing skills. Highly recommended for anyone aspiring to become an ISO 27001 Lead Auditor.