ISO/IEC 17025 is the internationally accepted standard that specifies the general requirements for the competence, impartiality, and consistent operation of testing and calibration laboratories. NABL accreditation to ISO/IEC 17025 confirms that a laboratory is technically competent and able to produce valid and reliable test and calibration results.
This standard applies to all laboratories performing testing, calibration, and sampling, regardless of size, scope, or industry sector.
NABL ISO/IEC 17025 accreditation demonstrates a strong commitment to technical excellence, measurement accuracy, and scientific integrity. It assures stakeholders that laboratory results are reliable, traceable, and generated under controlled conditions by competent personnel using validated methods and calibrated equipment.

NABL ISO/IEC 17025 accreditation confirms that laboratories:
By working with a NABL ISO/IEC 17025 accredited laboratory, clients and partners gain:
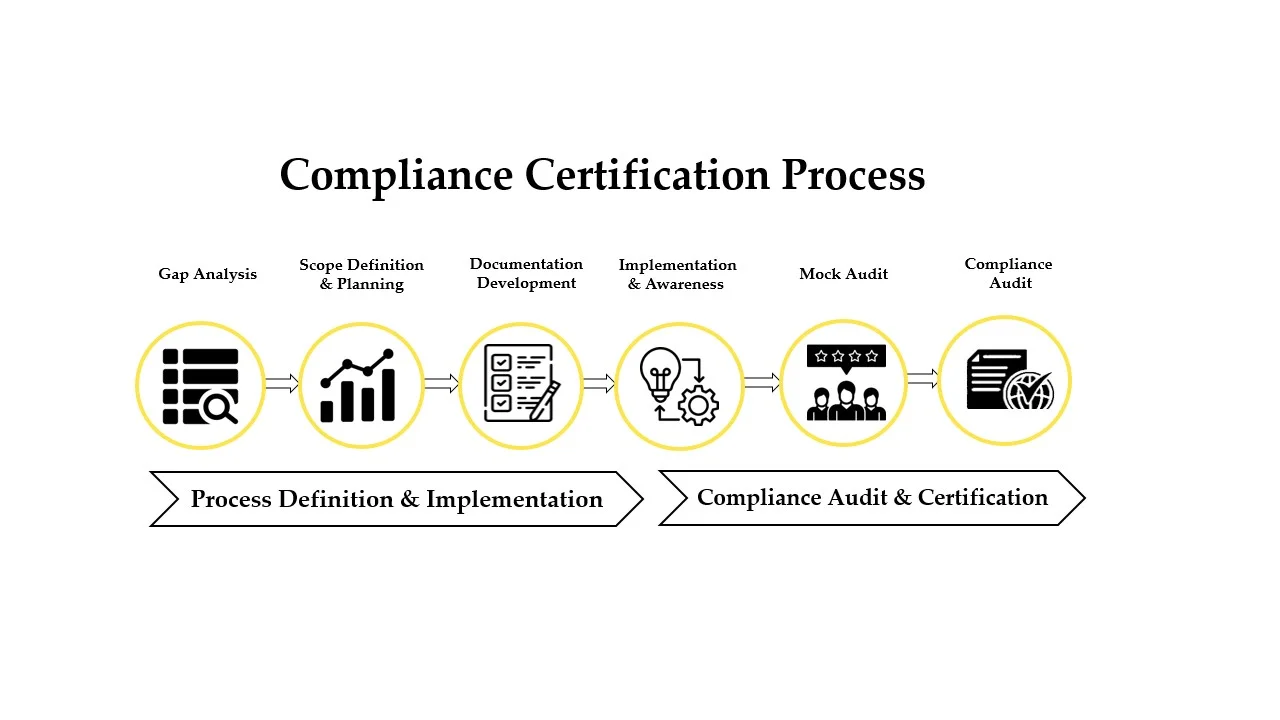
Strengthen credibility, meet international compliance standards, and build trust with customers and stakeholders through globally recognized ISO and compliance certifications.
TESTIMONIALS
Excellent training! The blend of theoretical knowledge and hands-on application elevated my auditing skills. Highly recommended for anyone aspiring to become an ISO 27001 Lead Auditor.