Good Distribution Practices (GDP) is an internationally recognized quality system for the storage, handling, transportation, and distribution of products across the supply chain. GDP defines requirements to ensure that products consistently maintain their quality, safety, integrity, and traceability from the point of origin to the end user.
GDP ensures that products are procured, stored, handled, and distributed under controlled conditions in accordance with applicable regulatory, statutory, and international guidelines, regardless of industry type.
GDP compliance certification demonstrates our strong commitment to maintaining product quality, safety, and reliability throughout storage and distribution activities. It reflects our dedication to structured processes, regulatory compliance, and risk-based controls that prevent damage, loss, contamination, mix-ups, or deterioration during distribution.
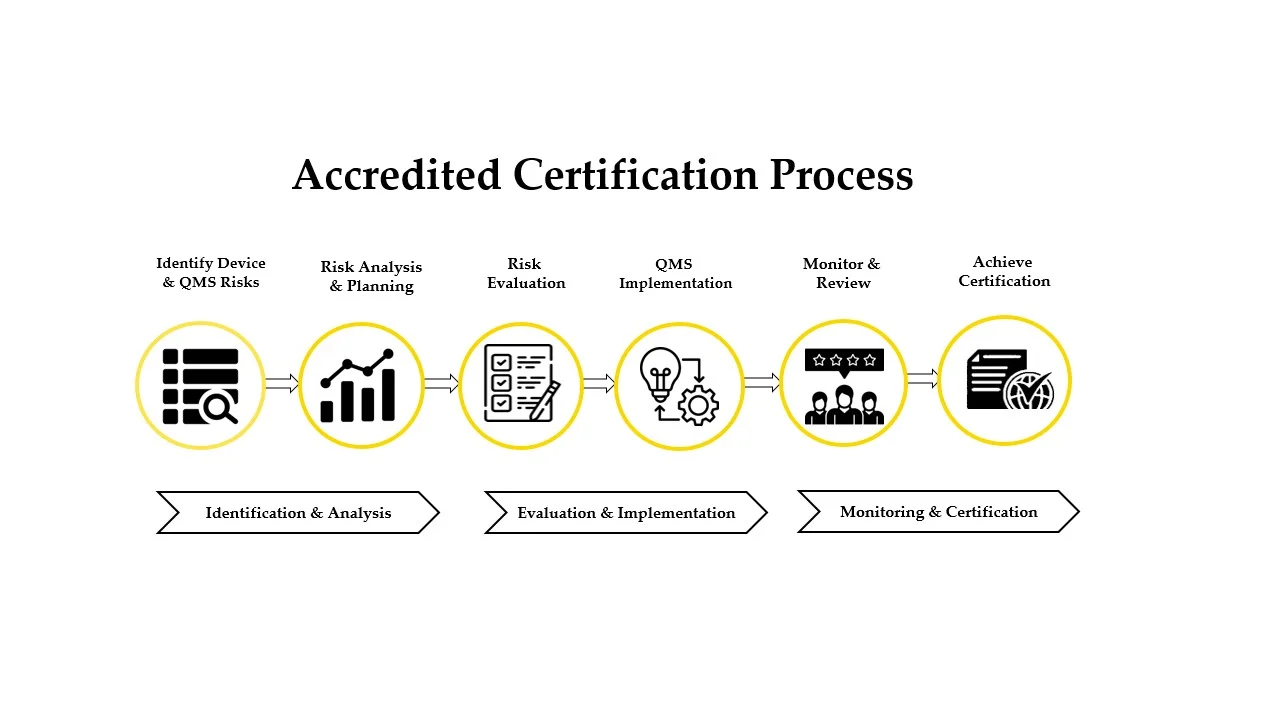
Through GDP implementation, we ensure defined procedures, trained personnel, secure facilities, monitored environments, and consistent distribution practices across all operational stages.

GDP compliance certification confirms that organizations:
By working with a GDP-compliant organization, clients and partners gain:
Strengthen credibility, meet international compliance standards, and build trust with customers and stakeholders through globally recognized ISO and compliance certifications.
TESTIMONIALS
Excellent training! The blend of theoretical knowledge and hands-on application elevated my auditing skills. Highly recommended for anyone aspiring to become an ISO 27001 Lead Auditor.